Quaternary Medium-Entropy Alloy Metallene with Strong Charge Polarization for Highly Selective Urea Electrosynthesis from Carbon Dioxide and Nitrate
Yuanbo Zhou1,2, Mengfan Wang4(王梦凡)*, Lifang Zhang3, Najun Li1, Tao Qian3, Chenglin Yan4,5(晏成林)*, Jianmei Lu1(路建美)*
1College of Chemistry Chemical Engineering and Materials Science, Soochow University, Suzhou 215123,P. R. China
2School of Optical and Electronic Information, Suzhou City University, Suzhou 215104, P. R. China
3School of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, P. R. China
4College of Energy, Key Laboratory of Core Technology of High Specific Energy Battery and Key Materials for Petroleum and Chemical Industry, Soochow University, Suzhou 215006, P. R. China
5School of Petrochemical Engineering, Changzhou University, Changzhou 213164, P. R. China
ACS Nano 2025, 19, 7273–7282
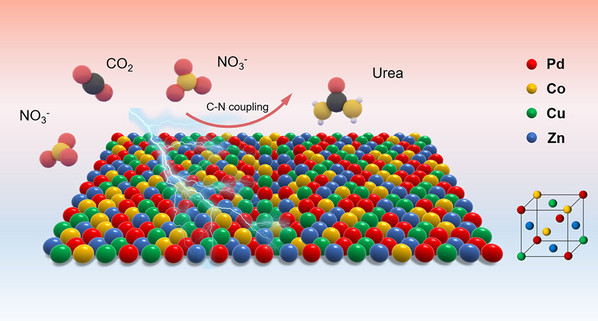
Abstract:Electrochemical urea synthesis via the coreduction of CO2 and NO3– is a sustainable alternative to the traditional Bosch–Meiser process. However, the sluggish reaction kinetics usually result in a low efficiency. Herein, we designed a kind of quaternary PdCuCoZn medium-entropy alloy (MEA) metallene for highly selective urea electrosynthesis. The random occupation of Cu, Co, and Zn with lower electronegativity in the face-centered cubic lattice of Pd-based metallene enables abundant electron donation from transition metals to adjacent Pd atoms, leading to the formation of charge-polarized Pdδ−–Cu/Co/Znδ+ sites. Considering that the pivotal C- and N-intermediates, namely, *CO and *NH2, are electrophilic and nucleophilic, respectively, such strong charge polarization would greatly benefit their respective formation and stabilization. The stable adsorption with *CO bonded to electron-rich Pd-based sites and *NH2 bonded to electron-deficient Cu/Co/Zn-based sites is demonstrated by the combination of in situ characterizations and theoretical calculations. The proof-of-concept PdCuCoZn MEA metallene achieves a maximum urea yield rate of 1840 μg h–1 mg–1 and a high Faradaic efficiency of 70.2%, surpassing most of the reported state-of-the-arts. Our strategy proposed in this work is believed to enlighten the design of an effective catalyst used for multistep reactions.
Article information: //doi.org/10.1021/acsnano.4c17546