Construction of Benzoxazole and Isoquinoline Compounds via Base-Mediated Cyclization of Amino Acid Derivatives
Yilian Song1, Yilian Song1, Zechao Liu1, Chuangchuang Liu1, Jingyu Zhang2(张静宇)*, Yingsheng Zhao1(赵应声)*
1Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China
2College of Energy, Soochow Institute for Energy and Materials Innovations, Soochow University, Suzhou 215006, P. R. China
Org. Lett.2025, 27, 3060–3065
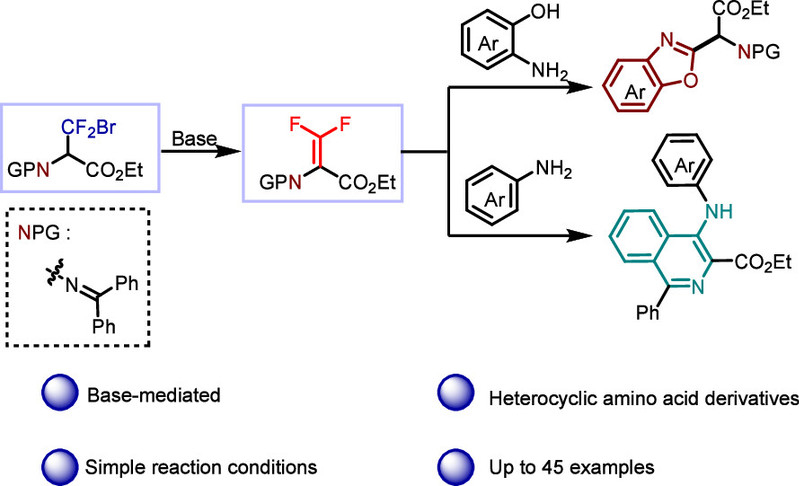
Abstract:Biological organisms contain bioactive macromolecules such as amino acids, which serve as basic materials for constructing cells and repairing tissues. Due to the unique properties of the fluorine atom, which can alter the structure of proteins and increase their lipophilicity, incorporating a fluorine atom into amino acids has become a research hotspot. In this study, ethyl 3-bromo-2-((diphenylmethylene)amino) -3,3-difluoropropanoate was synthesized from glycine derivatives. Under alkaline conditions, this compound reacted with 2-aminophenol to generate a benzoxazole-containing amino acid derivative. This method is simple to operate, without metal participation, and is performed under relatively eco-friendly reaction conditions, providing a novel approach for the synthesis of benzoxazole heterocycles.
Article information: //doi.org/10.1021/acs.orglett.5c00736