A General and Mild Two-Step Strategy Using Bioderived Diols and CO2 for Chemically Recyclable Polycarbonates and Closed-Loop CO2 Fixation
Jiaming Tian1, Nikos Hadjichristidis2, Xin Wang(王鑫)1*, Zhengbiao Zhang1,3(张正彪)*
1State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Materials, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
2Polymer Synthesis Laboratory, KAUST Catalysis Center, Physical Sciences and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal 23955, Saudi Arabia
3State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou 215123, China
Angew. Chem. Int. Ed. 2025, 64, e202423162
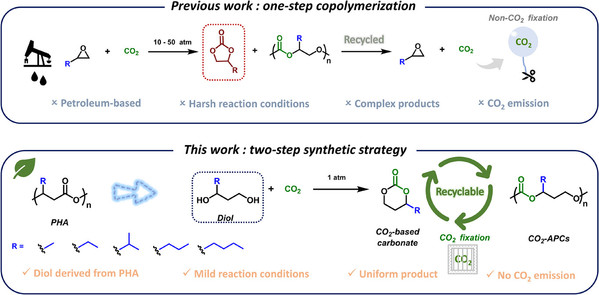
Abstract:Developing chemically recyclable polymers using CO2 and sustainable co-feedstocks is an important strategy for achieving carbon-neutral production of new polymers and mitigating plastic pollution. Herein, a series of six-membered cyclic carbonate monomers with different alkyl α-substituents were synthesized using CO2 and bioderived 1,3-alkanediol as raw materials at room temperature and atmospheric pressure. The organocatalytic ring-opening polymerization was systematically studied using a range of common and readily available organocatalysts. Phosphazene base (t-BuP2) was identified as the most effective catalyst, offering excellent control over the entire polymerization. The regioselectivity of the synthesized polycarbonates, ranged from 0.74 to 0.99, with the highest value achieved when the side group was isopropyl (highest steric hindrance). Notably, the α-substituent in the monomers reduced the ring strains, allowing the resulting polycarbonates to be fully recycled to the monomers without decarboxylation. The recycling process effectively traps CO2 in a closed loop between monomers and polymers, preventing its release into the atmosphere. The alkyl side groups enhanced the hydrophobicity of the polycarbonates, thereby reducing the likelihood of CO2 release through hydrolysis during their lifecycle, achieving a robust CO2 closed-loop fixation. The utility of CO2-based aliphatic polycarbonates as adhesives and the ability of copolymerization with l-lactide were explored.
Article information: //doi.org/10.1002/anie.202423162