Tuning the electrochemical redox-mediated mechanism of oxygen evolution on cobalt sites by hydroxide ion coupling
Wenjuan Song1, Xiaoyue Duan1, Poe Ei Phyu Win1, Xiang Huang3(黄祥)*,Jiong Wang1,2(王炯)*
1Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215006, P. R. China.
2Jiangsu Key Laboratory of Advanced Negative Carbon Technologies, Soochow University, Suzhou 215123, P. R. China
3Quantum Science Center of Guangdong-Hong Kong-Macao Greater Bay Area (Guangdong), Shenzhen 518045, China.
Chem. Sci.,2025, 16, 8889-8896
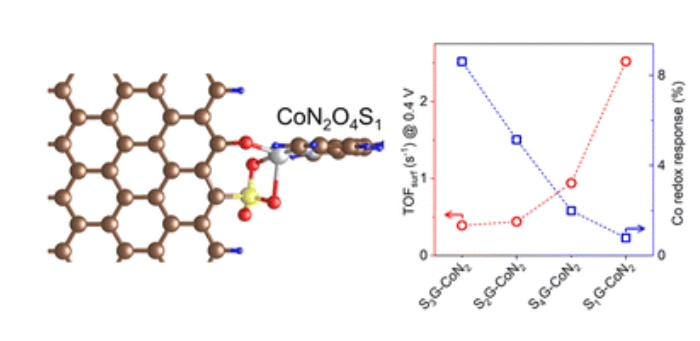
Abstract: Heterogeneous molecular catalysts (HMCs) with cobalt (Co) active sites are potent for the electrochemical oxygen evolution reaction (OER) in energy conversion applications. Such catalysts typically operate through the classical redox-mediated mechanism, where dynamic equilibria of Co2+/3+ and Co3+/4+ redox states are present before and throughout the OER cycle. However, the generation of low-valent Co2+ sites is disadvantageous for catalysis. To address this, sulfate groups embedded in graphene were developed to link a model Co-2,2′-bipyridine complex, resulting in the synthesis of a novel Co-based HMC that generates a specific CoN2O4S1 coordination moiety. These molecular Co sites were induced to oxidize from +2 to +3 oxidation state at open-circuit conditions, due to their proton-coupled electron transfer nature. This process ultimately eliminated the generation of the Co2+ state from its redox equilibrium and efficiently improved the turnover frequencies of Co sites toward OER, showing a two-order dependence on the concentrations of OH− ions. This work provides a novel mechanistic perspective for the rational design of high-performance HMCs.
Article information: //doi.org/10.1039/D5SC01674F