Crystallization and Melting Behaviors of Discrete Butyolactone–Caprolactone Alternative Co-Oligomer: The Effect of End Groups
Huijun Xu1, Ao Feng1, Zhihao Huang1, Rui Tan1(谭睿)*, Zefan Wang2(王泽凡)*, Xiaohua Zhang3,4,5(张晓华)*, Zhengbiao Zhang1(张正彪)*
1State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
2School of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, China
3Center for Soft Condensed Matter Physics and Interdisciplinary Research,Soochow University, Suzhou215006, China
4School of Physical Science and Technology and Jiangsu Key Laboratory of Frontier Material Physics and Devices, Soochow University, Suzhou215006, China
5Jiangsu Key Laboratory of Frontier Material Physics and Devices, Soochow University, Suzhou215006, China
Macromolecules 2025, 58,4579–4590
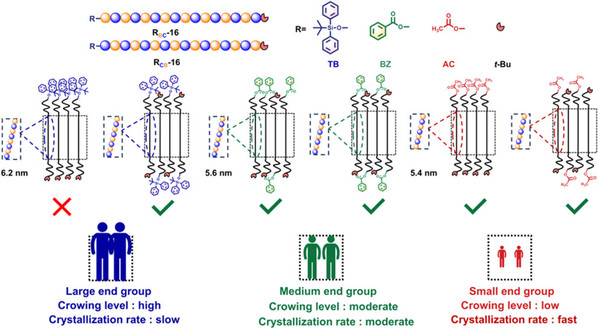
Abstract: Discrete oligomers are ideal candidates for the investigation of the crystallization behaviors of polymers since the molecular weight distribution and the precision of molecular structures play a crucial role in the crystallization behaviors of polymers. We precisely synthesize discrete alternative co-oligomers (o(γ-BL-alt-ε-CL)s) with different end groups based on ε-caprolactone (ε-CL) and γ-butyrolactone (γ-BL) as monomers using an iterative exponential growth (IEG) strategy and systematically study the crystallization and melting behaviors of the o(γ-BL-alt-ε-CL)s with precise monomer sequence. The roles that the end group plays in the crystallization and melting of co-oligomers are highlighted. The o(γ-BL-alt-ε-CL)s with different end groups (tert-butyldiphenylsilyl, benzoyl, acetyl) crystallize in an extended-chain conformation. Observation on the decrease of the crystallization rate of co-oligomers with the increase of the size of the end groups is associated with the slow dynamics or slow relaxation of co-oligomer chains with the large end group. The melting–recrystallization during the heating process for the o(γ-BL-alt-ε-CL)s with small end group is attributed primarily to the reduced steric hindrance effect. For the o(γ-BL-alt-ε-CL)s with large end groups, the rearrangement of co-oligomer chains is substantially hindered by steric effects, which lead to a significant suppression of the melting–recrystallization.
Article information: //doi.org/10.1021/acs.macromol.5c00593